Abstract
Background
Atypical hemolytic uremic syndrome (aHUS) is a rare disorder with an incidence of 0.23 to 1.9 per million population and presents with hemolytic anemia, thrombocytopenia and kidney failure. Eculizumab, a complement inhibitor and one of the most expensive drugs (about $26,000/dose), is the first specific treatment of aHUS. There is sufficient literature available regarding the use of Ecu in aHUS; however, practice conventions have not been well described.
Aims
The purpose of this study was threefold: 1) to describe patient demographics 2) report the clinical characteristics and laboratory outcomes 3) depict the outcomes of our cohort of patients (pts) treated for aHUS with Ecu.
Methods
A retrospective chart review was conducted to identify all pts age 18 and over who received 1 dose or more of Ecu for a diagnosis of aHUS/TMA and had an encounter at our tertiary academic center between 1/1/2017 to 7/30/2021. A linear mixed effects model was used with random effects for each pt and fixed effects corresponding to Ecu and a polynomial function of time since admission. Confidence intervals and p-values were calculated for the Ecu treatment effect on hemoglobin (Hg), platelets, lactate dehydrogenase (LDH), and creatinine (Cr).
Results
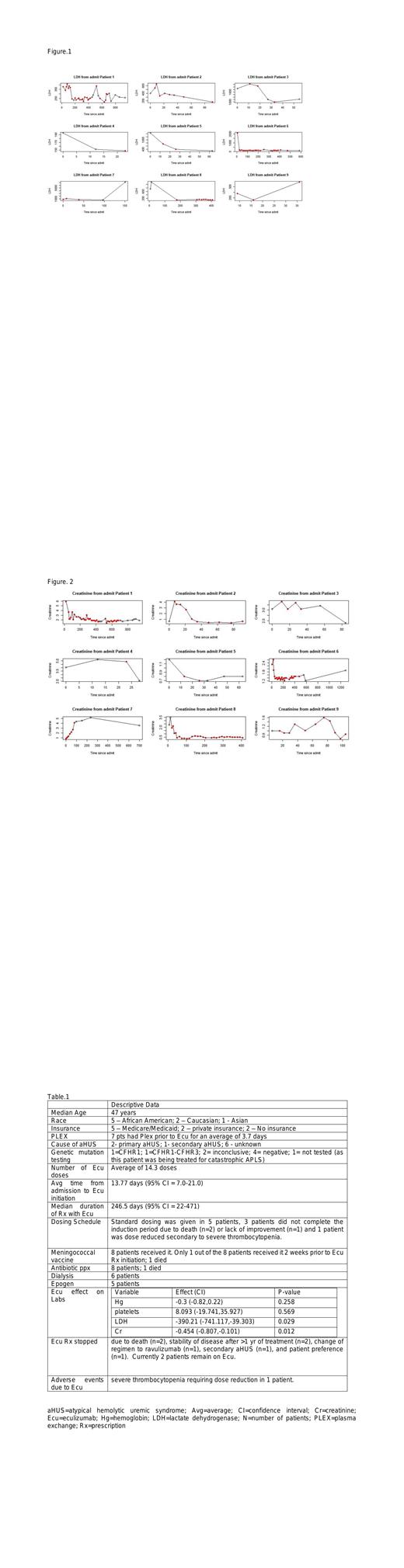
So far, 9 pts with aHUS/TMA treated with Ecu met criteria to be included in the study. Median duration of follow up was 12 months (1 - 51 months). The main findings are summarized in Table 1. Average time from admission to Ecu initiation was about 14 days. Most pts had received PLEX prior to Ecu. Only 1 out of 8 pts received meningococcal vaccination 2 weeks prior to initiation of Ecu. Pts were found to have a significant decrease in LDH (p=0.029; figure 1) and Cr (p=0.012; figure 2) while on treatment. No significant increase was found in Hg (p=0.258) nor was there a significant increase in platelets (p=0.569). 6 pts needed dialysis, but all of them were able to stop dialysis except the 2 pts who died. 1 pt did have interruption in treatment due to loss of insurance and 3 patients stopped treatment due to personal preference (n=2) or improvement in symptoms after more than a year on treatment (n=1).
Conclusions:
Ecu has been proven to be effective in pts with aHUS in clinical studies by resolving and preventing complement-mediated TMA, improving organ dysfunction and hematologic outcomes. Our study supported the available data by demonstrating an improvement in renal function and LDH, which is commonly used as a disease marker in aHUS. Optimal duration of Eculizumab therapy in aHUS has not yet been determined and current recommendations are to continue treatment indefinitely. Limited experience suggested that certain selected pts can be safely monitored off Ecu and prompt reinitiation of treatment on recurrence could be efficient in controlling the disease. Interestingly, our study found that all 3 patients who stopped treatment had stability of disease with sufficient follow-up. Also, the cost associated with Ecu use is a significant issue given the length of treatment currently recommended. Many of our patients did not have private insurance, which may influence the treatment patterns with Ecu. Lastly, just under half of our patients did not undergo genetic testing to determine the underlying cause of their aHUS presentation. Genetic mutations seem to be more clinically relevant to decide risk of relapse and to understand the pathophysiology of the disease. Improvement in these measures may help determine more information in their role in predicting clinical outcomes and treatment regimen duration.
Overall, more research is needed to elucidate the optimal treatment duration and investigate the role of various factors (such as insurance availability, genetic mutation testing, and patient preference) play in determining Ecu duration.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal